What is ISO 17025 standards?
ISO/IEC 17025:2017 is an international standard for quality management system that specifies the general requirements for the competence of testing and calibration laboratories.
This standard is widely recognized and accepted in many countries as a means of ensuring that laboratories have the necessary organizational and technical resources to produce accurate and reliable results for maintaining quality management system.
What is ISO 17025 and what is the objective or purpose?
This quality management system standard is widely recognized and accepted in many countries as a means of ensuring that laboratories which produce testing and calibration have the necessary technical and organizational resources to produce accurate and reliable results.
What are testing and calibration laboratories?
Testing laboratories and calibration laboratories are specialized facilities that conduct a range of tests and measurements to determine the characteristics, properties, or performance of materials, products, or systems. There are many types of testing and calibration laboratories.
Testing laboratories are facilities that perform a wide range of tests on various types of samples, such as raw materials, finished products, or environmental samples.
In simple word, Testing laboratories are organizations that produce testing.
Calibration laboratories, on the other hand, are facilities that has specialization with the calibration of measuring instruments and equipment.
Why is it important to calibrate your instruments with ISO 17025 Accredited lab?
It is important to calibrate instruments with ISO 17025 accredited lab because the standard provides a framework for the lab to demonstrate its competence and to ensure that calibration results are accurate and traceable to national or international standards.
Accreditation also means that laboratory is regularly audited by a third-party accreditation body, which further ensures the laboratory's technical competence and impartiality.
Additionally, using an accredited lab can be a requirement in certain industries or regulations, and can be beneficial for maintaining quality and consistency in measurements and results.
What is the difference between the ISO 9001 and the ISO 17025?
ISO 9001 is the standard for quality management systems, while ISO 17025 is specifically focused on the technical competence of testing and calibration laboratories.

ISO 9001 provides a framework for organizations to ensure that they are providing consistent products and services that meet customer requirements and expectations, while ISO 17025 provides a framework for laboratories to demonstrate their technical competence and to ensure the results they produce are accurate, reliable, and unbiased.
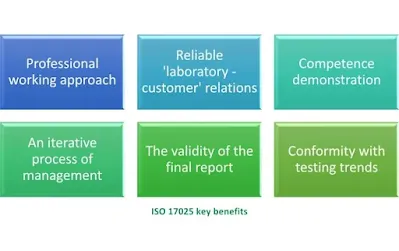
What are the benefits of ISO 17025 accreditation?
ISO 17025 accreditation provides a number of benefits for testing and calibration laboratories. Some of the key benefits include:
Improved technical competence:
This ISO standard provides a framework for laboratories to demonstrate their technical competence and to ensure the results they produce are accurate, reliable, and unbiased. This can help to improve the quality of the laboratory's work and increase customer confidence in results.
Increased efficiency:
By implementing the requirements for the competence of the ISO 17025 standard, laboratories can improve their overall efficiency and reduce the number of errors and rework. This can help to reduce costs and improve productivity.
Improved traceability:
ISO/IEC 17025 requires laboratories to have a system in place for traceability of measurements. This means that the laboratory's measurements can be traced back to national or international standards, which can improve the accuracy and reliability of results.
Enhanced reputation:
Accreditation to ISO/IEC 17025 demonstrates to customers, regulators, and other stakeholders that the laboratory is technically competent and that the results it produces are reliable and accurate. This can help to enhance the laboratory's reputation and increase its competitiveness in the market.
Compliance with regulations:
Many industries and regulations require the use of accredited laboratories, and accreditation to ISO/IEC 17025 can help laboratories to meet these technical requirements and the competence of testing and calibration laboratory to demonstrate compliance with relevant regulations.
Continual improvement:
The ISO standard requires regular internal and external audits, which can help to identify areas for improvement and to ensure that the laboratory is continuously improving its processes and services.
Is ISO/IEC 17025 Mandatory?
Accreditation to ISO/IEC 17025 is usually carried out by third-party accreditation bodies that are independent from a laboratory that produce testing and calibration and have been recognized by the relevant national accreditation body.
Accreditation to an ISO 17025 implies that the lab has been assessed by an independent body and found to meet the competence and technical requirements of the standard, which is a demonstration of for the competence of testing and calibration laboratories and impartiality.
Additionally, Some industries such as pharmaceuticals, food, and environmental, have regulations that require the use of accredited laboratories.
Also, some international regulations such as the CE mark in the European Union, require the use of accredited laboratories for certain types of testing and calibration.
In short, while ISO/IEC 17025 is not mandatory, it is highly recommended for laboratories that want to demonstrate their technical competence and to improve the quality and reliability of their results, and in some cases, it is a requirement for laboratories that want to participate in certain programs or to bid on certain contracts.
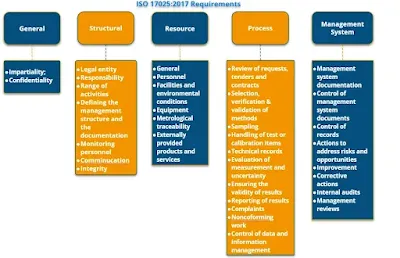
What are the Requirements of testing and calibration laboratory for ISO 17025 ?
ISO/IEC 17025:2017 is divided into several clauses in order to meet general requirements for the ISO/IEC 17025, each of which addresses a specific aspect of laboratory management system requirements and operations. The clauses and their main subclauses are:
General requirements for the ISO/IEC 17025:
This clause covers the general requirements for the competence, impartiality, and consistent operation of laboratories. It includes subclauses on the laboratory's quality management system, management responsibility, and resource management.
Technical requirements of ISO/IEC 17025:
This clause covers the technical requirements for testing and calibration activities. It includes subclauses on the laboratory's technical processes, measurement traceability, test and calibration methods, and handling of test and calibration data.
Management of the laboratory:
This clause covers the management of the laboratory, including subclauses on the laboratory's management system, personnel, and infrastructure.
Assessing and managing the quality of testing and calibration results:
This clause covers the conformity assessment and managing the quality of testing and calibration results, including subclauses on the laboratory's quality control procedures, measurement uncertainty, process requirements and reporting of results.
Assessing and managing the validity of test and calibration results:
This clause covers the conformity assessment and management of the validity of test and calibration results, including subclauses on the laboratory's procedures for assessing the validity of test and calibration results, and for handling test and calibration results that are found to be invalid.
Handling complaints and appeals:
This clause covers the laboratory's procedures for handling complaints and appeals, including subclauses on the laboratory's procedures for receiving, investigating, and resolving complaints and appeals.
Assessing the laboratory's performance:
This clause covers the laboratory's performance, including subclauses on the laboratory's internal and external assessments, and the laboratory's procedures for evaluating and reporting its performance.
Continual improvement:
This clause covers the laboratory's procedures for continual improvement, including subclauses on the laboratory's procedures for evaluating and reporting its performance, and for implementing corrective and preventive actions. These steps must be followed for accreditation of ISO/IEC17025
How to get ISO 17025 accredited?
ISO/IEC 17025 is the international standard for laboratory accreditation. To become ISO/IEC 17025 accredited, a laboratory must demonstrate that it operates at a high level of technical competence, and is able to produce accurate and reliable results. This process typically involves a thorough assessment of the laboratory's management system, as well as its technical processes and procedures.
What does ISO 17025 accreditation mean?
ISO 17025 accreditation means that a laboratory has been independently assessed and found to meet the requirements of the standard. It demonstrates that the laboratory is technically competent, and that it has a robust quality management system in place.
How many clauses are there in ISO 17025:2017?
ISO/IEC 17025:2017 has 10 clauses. These clauses are:
General requirements
Structure and responsibilities
Resources
Processes
Measurement traceability
Property management
Sampling
Testing and calibration
Handling, preservation and transport of test and calibration items
Reports and certificates
How to get ISO 17025 Certification in Nepal?
To obtain ISO/IEC 17025 certification in Nepal, a laboratory should follow these general steps:
Review the ISO 17025 standard: Familiarize yourself with the requirements of the standard and ensure that your laboratory meets all of the criteria.
Perform a self-assessment: Conduct a thorough self-assessment of your laboratory's management system, technical processes, and procedures to identify any areas that need improvement.
Implement any necessary changes: Address any gaps or non-conformities identified during the self-assessment by implementing changes to your laboratory's management system, technical processes, and procedures.
Contact an accredited certification body: Contact an accredited certification body, such as ISO Certification in Nepal Pvt. Ltd, that is authorized to certify laboratories to ISO 17025 in Nepal.
Schedule an assessment: Work with the certification body to schedule an assessment of your laboratory. The assessment will typically include a review of your laboratory's management system, as well as an on-site assessment of your laboratory's technical processes and procedures.
Achieve certification: If the assessment is successful, the certification body will issue an ISO 17025 certificate to your laboratory.
It's important to note that the process of getting ISO 17025 certification can be time-consuming, and it may take several months to complete. It is also important to note that the certification body should be accredited by an internationally recognized accreditation body, such as the International Accreditation Forum (IAF).
What should be the of accuracy as per ISO 17025?
ISO/IEC 17025:2017 states that the laboratory must demonstrate that its measurement results are of an appropriate level of accuracy. It is not specific about what the level of accuracy should be, but rather it leaves it to the laboratory to determine what is appropriate for the specific measurement being made, taking into account the uncertainty of measurement and the required level of confidence. The laboratory should establish, implement and maintain procedures for the evaluation of measurement uncertainty, to ensure that measurement results are accurate, traceable and comparable.
Frequently Asked Questions:
- Can
lab tests be inaccurate? Yes, lab tests can be inaccurate due to
various factors such as equipment calibration issues, human errors,
improper sample handling, environmental conditions, and outdated testing
methodologies. To minimize inaccuracies, ISO Certification in Nepal Pvt.
Ltd. follows strict quality assurance procedures, regular calibration of
equipment, and continuous training of lab personnel.
- How
to test cement in the lab? Testing cement in the lab involves various
standardized methods like compressive strength test, setting time test,
fineness test, soundness test, and chemical analysis. ISO Certification in
Nepal Pvt. Ltd. employs trained technicians and calibrated equipment to
ensure accurate and reliable cement testing.
- How
to test wine quality in the lab? Wine quality testing in the lab
includes analysis of various parameters like alcohol content, acidity, pH
level, color, aroma, and taste. ISO Certification in Nepal Pvt. Ltd.
adheres to international standards to assess wine quality, ensuring
consistency and credibility.
- Why
perform lab tests for deformability measurement? Lab tests for
deformability measurement are crucial to understand the material's
response to stress, strain, and load-bearing capacity. These tests aid in
product design, structural analysis, and material selection, ensuring
safety and reliability.
- Why
perform lab tests for rock deformability measurement? Lab tests for
rock deformability measurement are essential in geotechnical and mining
industries to assess rock strength, stability, and behavior under various
conditions. These tests help in designing secure structures and mitigating
geological hazards.
- How
can quality be achieved through testing? Quality can be achieved
through testing by utilizing reliable testing methods, calibrated
equipment, trained personnel, adherence to international standards, and
robust quality assurance practices. Regular monitoring and corrective
actions further enhance the quality of testing processes.
- What
are the quality assurance standards in a lab? Quality assurance
standards in a lab include ISO/IEC 17025:2017, which sets out general
requirements for the competence, impartiality, and consistent operation of
testing and calibration laboratories.
- What
is included in quality assurance? Quality assurance includes
procedures for quality control, equipment calibration, training and
competency assessment of staff, maintaining proper documentation, regular
audits, and continuous improvement initiatives.
- Why
is quality assurance necessary in different organizations? Quality
assurance is essential in different organizations to ensure the accuracy
and reliability of their products or services. It builds customer trust,
meets regulatory requirements, and leads to continual improvement.
- Why
is quality control and quality assurance important? Quality control
ensures that products or services meet predetermined standards, while
quality assurance ensures that the processes leading to those products or
services are consistent, reliable, and continuously improved.
- How
can quality assurance be achieved? Quality assurance can be achieved
by implementing and following standardized procedures, maintaining
well-calibrated equipment, conducting regular internal audits, providing
training to staff, and addressing any non-conformities.
- How
to do quality assurance? To do quality assurance, establish quality
policies and procedures, implement process controls, monitor and measure
performance, conduct regular internal audits, and take corrective actions
to improve processes.
- How
to implement quality assurance? To implement quality assurance, create
a quality management system based on international standards, define clear
quality objectives, assign responsibilities, and communicate the
importance of quality to all staff.
- How
is data quality assured? Data quality is assured through validation
checks, proper data entry procedures, regular data audits, and using
reliable data sources. ISO Certification in Nepal Pvt. Ltd. ensures data
accuracy and integrity to provide credible results.
- Why
do calibration in the lab? Calibration in the lab ensures that
measurement equipment is accurate and reliable, providing precise and
consistent results. Regular calibration is vital for maintaining the
quality of testing and measurement processes.
- What
is verification and validation? Verification ensures that a product,
process, or system meets specified requirements. Validation, on the other
hand, confirms that the product or process fulfills its intended use in
the real environment.
- How
can observations be made more valid and reliable? To make observations
more valid and reliable, use standardized observation methods, minimize
bias, conduct multiple observations, involve trained observers, and
validate the observations against other sources of data.
It is important to work with a reputable ISO consultant like ISO Certification in Nepal Pvt. Ltd. that can provide cost effective expert guidance and support to your organization throughout the process of ISO 9001 implementation. We are the leading ISO System Certification body in Nepal. We will help an organization to understand requirements, implement the standard and ensure that your company is in compliance with any ISO Certification in Nepal. Besides to ISO 9001 quality management system, we also provide ISO 14001( Environmental management System certification) , ISO 22000(Food Safety Management System Certification) ISO 45001 Occupational health and safety management system , ISO 27001 Information Security Management System . ISO/IEC 17025 Testing and calibration laboratories Organic Certification in Nepal. If you have any queries as well as if you want to get your organization to be ISO Certified, please call 9840525565 to get free consultation on ISO Certification service and know which ISO standards is best for your organization.